James Harrison

Research
Quantum Chemistry
Advances in fundamental theory and computer technology enable us to construct wavefunctions for atoms and molecules which are of unprecedented accuracy. We use these functions to assist in the interpretation of spectroscopic experiments and to develop and refine the qualitative notions of chemical bonding. Our current focus is to understand:
The electronic structure of the ground and low-lying states of small molecules containing a transition metal atom. Diatomics of interest include MX where M is a transition metal (Sc to Zn) and X is a main group element (H to Cl). These molecules are of great interest as models for the nature of the transition meta-main group element chemical bond. Triatomics include the metal hydroxides MOH & HMO and the carbynes MCH, the understanding of which is fundamental to the reactions of transition metals with hydrocarbons. We are also interested in the structure of the mono- and dipositive ions of these systems.
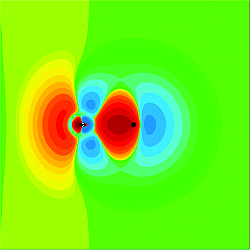
The nature of molecular multipole moments and the information contained in these moments
about the chemical bond. While we all have an instinctive feeling about the meaning
of a molecular dipole moment and how it reflects the charge distribution in a molecule
the same instincts often fail when considering for example, the quadrupole moment.
Some of this problem is that the quadrupole moment is a second rank tensor while the
dipole moment is a tensor of the first rank. However even for homonuclear diatomics
where the quadrupole tensor has only one unique component the relationship between
this component and the molecular charge density is not well understood. We have recently
shown that the molecular quadrupole moment can be written as the sum of the quadrupole
moments of the constituent atoms plus a term that depends on the shift in the electron
density upon bond formation. In the course of this work we have defined the quadrupole
moment density that shows where in the molecule the molecular contribution to the
quadrupole moment comes from. We are extending these ideas to other one electron properties
like the electric field gradient at a particular nucleus and the dipole moment (still
more to learn!).
The spatial distribution of electron spin in open shell molecules. We have recently shown in the open shell nitrogen halides, NF, NCl and NBr that α and β spins flow in opposite directions as the chemical bond forms. We are exploring this observation and the role that electronegativity (chemical potential) plays.